May 11 marked the end of the federal COVID-19 public health emergency. Locally produced online dashboards have been offline since March and even the Centers for Disease Control and Prevention ceased to produce its weekly COVID data tracker.
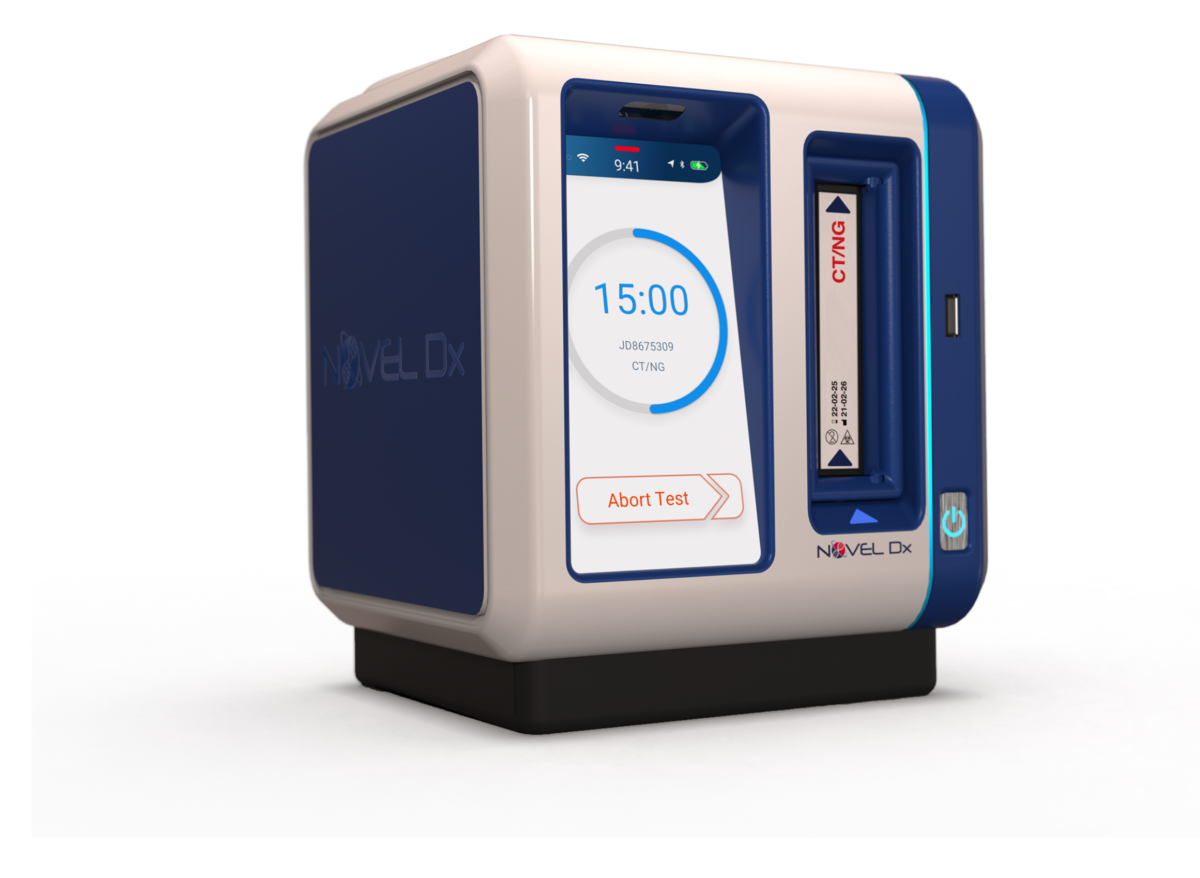
That doesn’t mean cases are nonexistent, though. To that end, biotech and diagnostic company Novel Microdevices is still working to fine-tune its PCR testing technology. The East Baltimore-based company’s focus on improving diagnostic capabilities is further enabled by its recent receipt of a $1.4 million Phase 1 award from the National Institutes of Health’s (NIH) Rapid Acceleration of Diagnostics (RADx) program. This funding will support the company’s development of its rapid point-of-care RT-PCR platform.
Andrea Pais, the founder and CEO of the East Baltimore-based medical device developer, is confident in the technology’s market case and medical intervention.
“Our technology is unmatched in the market, providing highly accurate, rapid, and affordable diagnoses at point-of-care and near-patient settings, enabling immediate treatment initiation,” she said.
At its core, Novel Microdevices’ groundbreaking technology takes intricate scientific methods performed by high-complexity labs and condenses the processing time into a portable device.
Leveraging the recent NIH-RADx funding, Pais elaborated on the company’s strategic intentions, stating: “The funding infusion will significantly aid Novel Microdevices in finalizing product development, with the aim of expediting manufacturing scalability and initiating crucial clinical validation for regulatory clearance.”
Pais went on to explain the distinctive advantages of Novel Microdevices’ rapid PCR diagnostic technology for respiratory viruses.
“Our PCR-based solution empowers instant diagnosis of infectious diseases in accessible patient settings, facilitating on-site treatment initiation and curtailing potential disease transmission,’ she said. “This eliminates the need for prolonged waiting periods for results from high-complexity laboratories.”
This award is the latest in a series of major funding moves for the company, including a $13.8 million CARB-X award in 2021 and a $5 million raise earlier this year.
Before you go...
Please consider supporting Technical.ly to keep our independent journalism strong. Unlike most business-focused media outlets, we don’t have a paywall. Instead, we count on your personal and organizational support.
Join our growing Slack community
Join 5,000 tech professionals and entrepreneurs in our community Slack today!

The person charged in the UnitedHealthcare CEO shooting had a ton of tech connections

The looming TikTok ban doesn’t strike financial fear into the hearts of creators — it’s community they’re worried about

Where are the country’s most vibrant tech and startup communities?