An acquisition wasn’t the only milestone this year for a local team working on technology to help patients continue to breathe when their lungs aren’t working properly.
The Breethe system, which was initially developed by a surgeon at Baltimore’s University of Maryland School of Medicine, received clearance from the U.S. Food and Drug Administration late last month.
Medical device company Abiomed said in October that the system, which is designed to behave like a human lung, has received the regulatory clearance that allows it to be marketed in the U.S. FDA clearance is often a key hurdle that allows companies to begin selling, and Breethe has completed all testing required before going to market. The product will be introduced in the U.S. through a “controlled rollout” to hospitals, an Abiomed spokesperson said.
The FDA clearance comes six months after Danvers, Massachusetts-based Abiomed acquired Breethe, which was founded by UM School of Medicine surgeon and professor Dr. Bartley Griffith. Abiomed maintained Breethe’s 24-person team, which is based in Halethorpe, as it continued to develop the device.
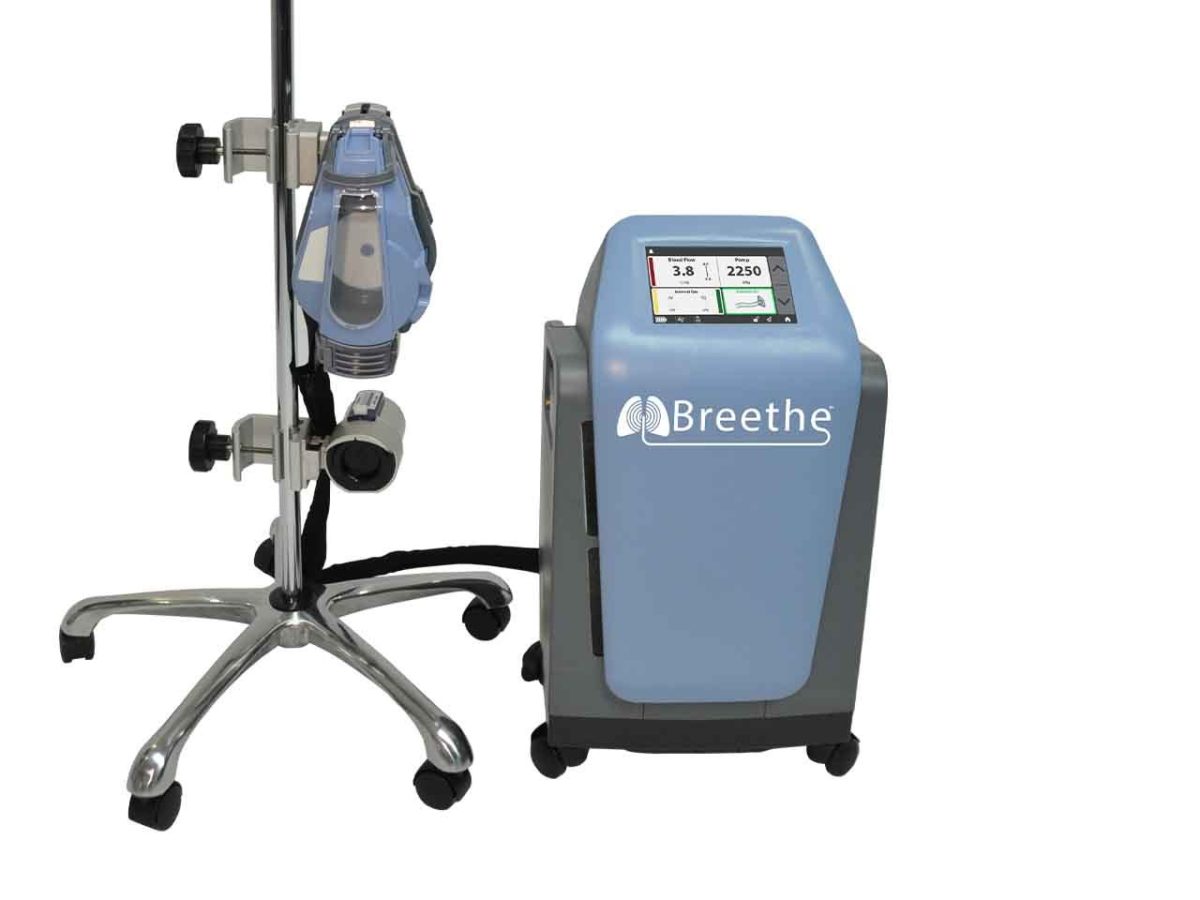
Breethe’s system is designed for use in ECMO therapy, which is a form of life support. The device, called the Abiomed Breethe OXY-1 System, is designed to pump oxygen to blood and remove carbon dioxide when lungs can no longer function properly. The FDA clearance allows the device to be used for up to six hours. The company said it can be used when patients experience respiratory failure while battling diseases such as ARDS or COVID-19.
The device is designed to be portable, so a patient does not need to be stationary in a bed to use it, and Abiomed said it’s compact, and intended to be moved freely.
Abiomed also makes a heart pump product, which could be used in tandem with the Breethe system.