Updated 9/18/09 @ 2:42 p.m. Name in title
When Jane Hollingsworth takes a pill to help fight a headache, she might get nauseous or sicker still.
More than half of American adults suffer similarly, she says, which is a bear of a nuisance for anyone with an acute migraine and a problem with the most common medicinal cure. Still many just put up with the pain.
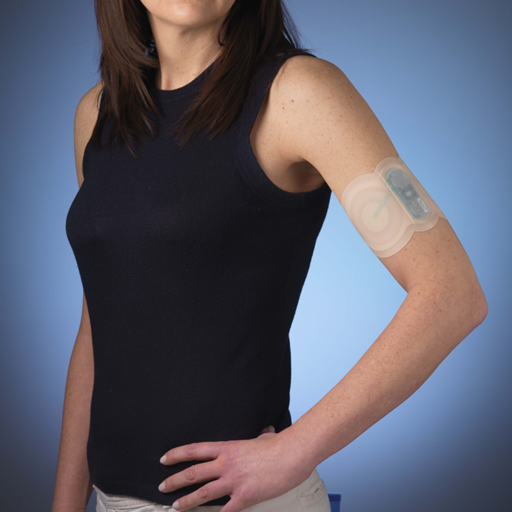
Because it’s affecting millions of people who just might happily pay for a solution, there is admitted industry buzz swarming NuPathe, the Conshohocken-based specialty pharmaceutical company that says it could help everyone with a pain in their head who doesn’t want a pill to swallow. After a scheduled new drug application is filed next year, you just might know someone who uses Zelrix, a NuPathe-manufactured patch that secretes migraine-fighting medication into the bloodstream.

“There is no patch for migraines now. There has never been,” says Hollingsworth, 50, the 25-employee company’s CEO who helped launch it in 2005. “It’s very difficult to get drugs through the skin quickly, which is important for migraines especially.”
Difficult for everybody else, she must mean. Because, as the company’s comprehensive phase-III trial data summary presentation suggested at last week’s 14th Congress of the International Headache Society held at the Convention Center, things for NuPathe are going, as Hollingsworth says, “exceedingly well.”
After the jump, the Ardmore native tells us how technology makes Zelrix work, why biomedical entrepreneurship in Philadelphia lags behind smaller hubs like Boston, why she has to cheer for the Flyers and more.
Interview edited for length and clarity.
Explain the technology behind Zelrix.
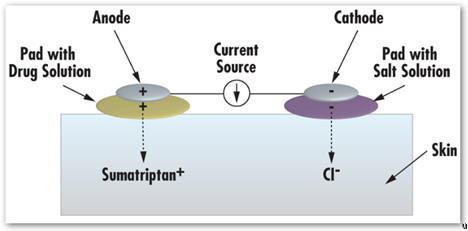
We’re using [migraine medication] sumatriptan and an anthretic device, which means an exchange of ions. It’s a positively-charged drug moving with a salt solution.
The device is powered by a very small, almost-like watch batteries. You push the button, and it starts an exchange of ions, positive chasing negative. It’s iontophoresis, our [proprietary] iontophoretic transdermal technology [known as SmartRelief].
So why is the patch so necessary?
With the device, we can deliver the medication very quickly and control that deliverly for as long as we want. [We’re using] a four-hour patch, meaning you don’t have to swallow a pill, which will be comforting to the millions of people who can get nauseous from an oral treatment, myself included.
Because we control how much drug gets into the blood stream, far more than a pill, for example, there are lower side effects, the Triptan side effects, chest tightness and panic, tightness of the neck and these other problems many people face when taking migraine medications today.
While totals and rounds of investment in the privately-held pharma startup are not disclosed, a handful of leading healthcare venture capitalists have made bets on the future of the company Hollingsworth co-founded with Terri Sebree in 2005.
Below are seven firms that are known to have made investments in NuPathe:
Sometimes you shoot� high because you’re going into work and want to stave off the pain. Now with a non-oral solution, we control it.
According to our data, under two percent of our users have Triptan effects. …That can be compared with an oral average of 10 percent, or the other non-oral solution, injection is in nearer to 40 percent.
What’s next in the uphill slog to getting Zelrix to market and what other work is NuPathe doing?
Well, now we wait some. We’re waiting for some more long-term data to be completed sometime next year. Then we submit [a new drug application with the Food & Drug Administration]� to get marketing approval.
…In terms of other work, we have NP 201, which is a Parkinson’s disease product. We’re still getting data for our research on our work. But now in these earlier stages, we have high hopes for the product. I hope to hear something exciting within the next month or so.
Tell us something about you that has nothing to do with science.
Well, my husband and I have two boys and we live in Fort Washington. [laughs] So, I’m surrounded by male pursuits, ice hockey and the� Flyers particularly. So, yes, we are sports fans. …There’s a restaurant we like called Allison Two. It’s a good one, and there aren’t as many in a place like Fort Washington as, say, on the Main Line.
Tell us about your ties to the region.
Well, I was born and raised in the region… in Ardmore.
It’s as good a place to live as any, especially being from this particular industry. My father worked his entire career, nearly his entire life as general counsel for SmithKline. I spent my life hearing about pharma.
It’ an exciting place. There are a� lot of resources. You can start a company in a place like San Francisco more easily and maybe get more funding, but when you talk about building a company, nowhere else are there the commercial resources and other assets.
When we wantd to launch NuPathe, we did look around about where to start, but we decided what we needed, what is hardest to find, is the people. Money is fungible. Science is fungible, but this is where you have the smart people in the biomedical fields.
Now that you’ve brought that up, last month I spoke to Russel Grieg, the CEO of SR One, GlaxoSmithKline’s biomedical venture capital firm which has invested in NuPathe. He said he has “always been disappointed” by the level of biomedical entrepreneurship in Philadelphia, though he credited NuPathe with being an exception.
Why do you think Philadelphia, which has such a reputation for pharmaceutical and health care development, lags behind in biomedial innovation?
One of the pros and cons in this region is we have a lot of big pharma companies. For a comapny like us, that means there’s a large talent pool. That’s great. But wha’ts made more difficult for entrepreneurship is that folks tend to find their way into big companies. The talent tends to be fed into big companies here, so they aren’t out doing things on their own, vying for funding to start out on their own.
I do see that evolving. As a region we’ve gotten much more cohesive. State funding in this area does work, but the industry needs to do better to work together to help each other to use their collective resources to spawn new companies, to help the region and the industry evolve more. You see this contraction, like Wyeth and Pfizer merging, and with this combination, well, maybe there will be more collaboration and a more startup mentality [in the Philadelphia region’s biomedical sectors].
…I’d like to see that happen.
-30-
Every Friday, Technically Philly brings an interview with a leader or innovator in Philadelphia�s technology community. See others here.






