After nearly a decade in development, a device to treat breast cancer that was developed at the University of Maryland School of Medicine has a key clearance toward going to market.
The U.S. Food and Drug Administration gave a 510(k) medical device clearance to the GammaPod, a device which treats early-stage breast cancer. The announcement was made in December.
First Stereotactic Radiation Therapy System Designed to Treat Early Stage Breast Cancer Receives FDA Clearance https://t.co/jrhWPQwK5R @UMmedschool @UMBaltimore pic.twitter.com/BhA7pCzVpB
— UM Ventures (@UMVentures) January 5, 2018
“During radiation therapy, tumor cells are killed when their DNA is damaged by the radiation being absorbed into them. While radiation therapy has the potential to kill tumor cells, it can also damage healthy tissue around the tumor,” the FDA stated.
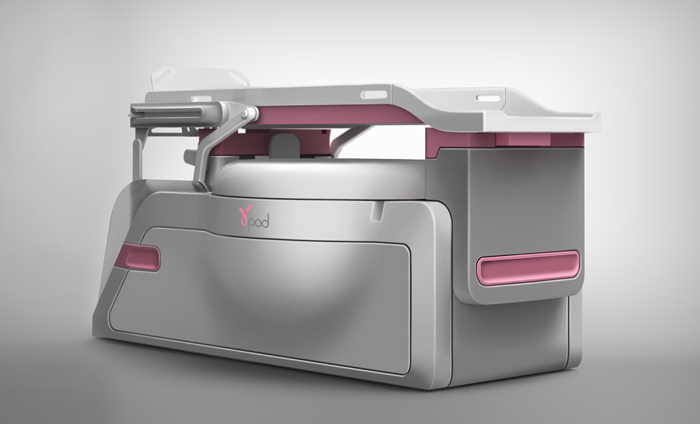
GammaPod requires patients to lie on their stomach. According to the University of Maryland-Baltimore, the device delivers a high dose of radiation to a tumor, but keeps radiation from getting to normal breast tissue and other organs.
“GammaPod uses thousands of focused beams of radiation from 36 rotating radioactive Cobalt-60 sources in combination with a two-layer, vacuum-assisted cup that immobilizes the breast to achieve a more accurate delivery of radiation,” the FDA states.
It is not designed to replace Whole Breast Radiation Therapy, but the device could also reduce the time of the more targeted treatments from many weeks to less than a week.
“The GammaPod delivers a uniform dose to the tumor but the amount of radiation drops off rapidly outside the targeted area with a substantially reduced dose to healthy breast tissue,” Elizabeth M. Nichols, an assistant professor of radiation oncology at UMSOM who led a feasibility and safety study of the device in 2016, said in a statement. “We believe that this reduced exposure will result in better cosmetic outcomes for patients.”
The path for the device to date shows one local pathway toward bringing such a device to market. The GammaPod was invented by scientists at the University of Maryland School of Medicine in Baltimore including radiation oncology professor William F. Regine. The prototype was tested at the University of Maryland Medical Center. Columbia-based Xcision Medical Systems handled manufacturing, and applied for the FDA clearance to bring the device to market.
While clinical trials will continue to investigate further uses, the first treatment is expected to be offered at UM Medical Center in spring 2018.
Join the conversation!
Find news, events, jobs and people who share your interests on Technical.ly's open community Slack

Baltimore daily roundup: Mayoral candidates talk tech and biz; a guide to greentech vocabulary; a Dutch delegation's visit

Baltimore daily roundup: Medtech made in Baltimore; Sen. Sanders visits Morgan State; Humane Ai review debate

Baltimore daily roundup: An HBCU innovation champion's journey; Sen. Sanders visits Morgan State; Humane Ai review debate